Introduction
Tafasitamab (MOR208) is a CD19-targeting, Fc effector function-enhanced antibody that has shown promising clinical activity in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL). For patients newly-diagnosed with DLBCL, R-CHOP regimens combining the anti-CD20 antibody, rituximab (RTX) with chemotherapy is the standard of care; however, 30-40% of patients experience relapse with a high mortality rate.
CD20 is not as broadly expressed as CD19 on B-cell lymphomas, and CD19 expression is preserved on CD20-negative tumor sub-populations and following CD20-targeting therapy (Horna, et al. EHA 2020). These findings support the evaluation of immunotherapies combining anti-CD19 and anti-CD20 antibodies to target all malignant lymphoma cells.
To further explore the rationale for adding tafasitamab to a RTX-containing regimen, we analyzed the effect of this combination on antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), and tumor B-cell viability in vitro. Additionally, protein expression of the oncogenic transcription factor c-Myc was investigated to gain deeper insight into the molecular effects mediated by RTX and tafasitamab.
Methods
A comprehensive panel of 11 aggressive lymphoma cell lines was analyzed (9 DLBCL and 2 Burkitt's lymphoma). For the ADCC assays, tumor cells were incubated with tafasitamab and/or RTX at saturating concentrations in the presence of natural killer cells from healthy human donors at effector-to-target (E:T) ratios of 0.5:1 to 3:1 for 2 hours. Cytotoxicity was assessed by quantification of DAPI-positive, CFSE-labeled target cells. For the ADCP assays, lymphoma cells were incubated with tafasitamab and/or RTX at saturating concentrations in the presence of in vitro differentiated macrophages derived from healthy human donors at an E:T ratio of 2:1 for 3 hours. Phagocytosis was assessed by quantification of double-positive events (CFSE-labeled macrophages and CellTrace Violet-stained target cells). C-Myc protein levels were measured following the treatment of tumor cells with tafasitamab and/or RTX for 17-48 hours. CD19/CD20 surface expression, ADCC and ADCP rates, as well as intracellular c-Myc protein levels were analyzed by flow cytometry. Direct effects of antibody treatment on cell viability were assessed by the determination of ATP levels upon incubation of lymphoma cells for 24-96 hours.
Results
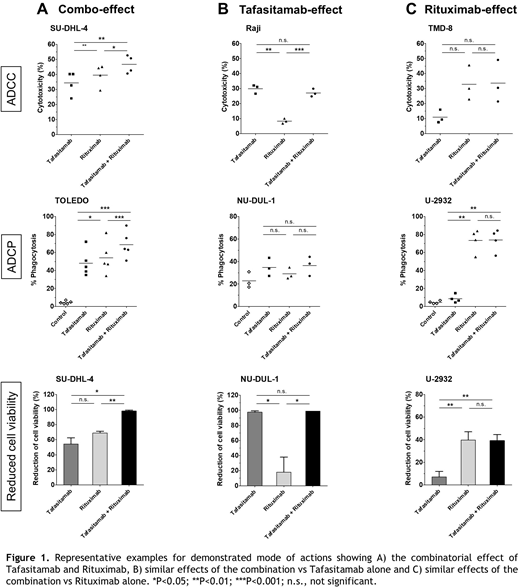
Increased ADCC activity for the combination of tafasitamab and RTX compared with the respective monotherapies was observed in 4/11 cell lines. For the remaining cell lines, the combination activity was similar to the most active monotherapy (tafasitamab: 5/11, RTX: 2/11).
Furthermore, in 5/11 cell lines, the combination of tafasitamab and RTX resulted in enhanced ADCP activity compared with the monotherapies. Similar to ADCC, the combination activity in the other cell lines was comparable to the most active monotherapy (tafasitamab: 2/11, RTX: 4/11). Interestingly, a slight correlation between ADCC/ADCP activity and CD19/CD20 surface expression levels was observed.
Treatment of tumor cells with the respective antibodies without effector cells showed a beneficial combinatorial effect of tafasitamab with RTX on the reduction of cell viability in 3/8 cell lines, whereas 2 cell lines were primarily sensitive to tafasitamab and 3 were sensitive to RTX. This observed difference in the activity profile of tafasitamab versus RTX suggests different mechanisms for induction of direct cytotoxicity (Figure 1).
To further elucidate the molecular basis of the observed direct effects on cell proliferation, expression levels of c-Myc were assessed. A correlation between decreased cell viability and reduction of c-Myc expression was demonstrated. This finding is in line with the role of c-Myc in the regulation of cellular processes, such as proliferation and apoptosis.
Conclusions
The co-treatment of tafasitamab with RTX demonstrated superiority compared with the respective monotherapies for at least one and up to all three of the modes of action tested (ADCC, ADCP, or direct impact on cell viability) in 9/11 lymphoma cell lines. These data demonstrate that the combination of tafasitamab and RTX mediates increased tumor cell death in vitro via different mechanisms of action and support the clinical evaluation of this antibody combination in patients with DLBqCL.
Patra:MorphoSys AG: Current Employment. Augsberger:MorphoSys AG: Current Employment. Ginzel:MorphoSys AG: Current Employment. Polzer:MorphoSys AG: Current Employment. Landgraf:MorphoSys AG: Current Employment. Bartel:MorphoSys AG: Current Employment. Ness:MorphoSys AG: Current Employment. Steidl:MorphoSys AG: Current Employment. Heitmüller:MorphoSys AG: Current Employment. Schanzer:MorphoSys AG: Current Employment. Endell:Morphosys: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal